
发布时间:2017-11-24| 作者:admin
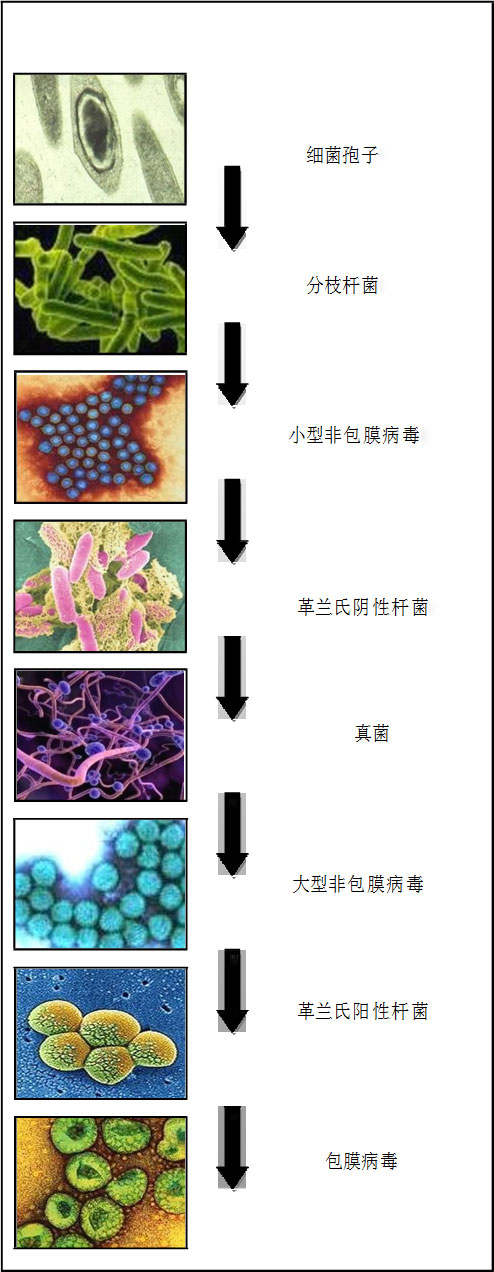
过氧化氢蒸汽(HPV)作为一种生物消毒剂因其对微生物具有广谱杀菌及快速灭活的功效已 在全球各国家/地区得到广泛应用。HPV灭菌的特点是 无残留(其分解产物只有水和氧气 )和仅需较低的环 境温度,蒸汽发生阶段增加了灭菌过程的实用性。
HPV已经对许多生物及不同级别的生物进行了灭菌测 试,实验证明:HPV对包括细菌、病毒和真菌在内的 微生物均具有广谱杀菌的功效。 HPV对于普遍存在于 环境表面的细菌孢子灭杀的有效性经验证是可以反复进 行的,因此,它位于Spaulding分类中的第一位。文件 中列出的有机体是按广义分类的(例如细菌、病毒和
实验证明,利用过氧化氢蒸汽(HPV)对微生物进行 消毒灭菌可达到6-log的灭杀率。
1. 经测试的生物及参考文献/出处
1.1 细菌和细菌孢子
|
生物类型 |
名称 |
参考文献/出处 |
|---|---|---|
|
细菌孢子 革兰氏阳性杆菌 |
炭疽芽孢杆菌 蜡样芽胞杆菌 环状芽胞杆菌 强固芽胞杆菌 巨大芽胞杆菌 短小芽胞杆菌 枯草芽孢枉菌 肉毒梭菌 艰难梭菌(难辦梭菌) piliforme梭菌 产芽孢梭状芽孢杆菌 嗜热脂肪芽孢杆菌 |
(1,2) (3,4) (3) (3) (3) (3,6) (1,3,6-8) (9) (10,11) (12) (6-8) (1,6,7,9,13,14) |
|
革兰氏阳性细菌 |
包皮垢分支枉菌 结核分枝杆菌 caesei乳酸杆菌 单核细胞增多性李司忒氏菌 |
(6) (13) (6) (4) |
|
革兰氏阳性球菌 |
屎肠球菌(简称VRE) 金黄色酿脓葡萄球菌(简称MRSA) 表皮葡萄球菌 |
|
|
肠杆菌科 |
阴沟肠杆菌 大肠杆菌 (简称O157:H7) 克雷白氏杆菌 猪霍乱沙门氏菌 灵杆菌 鼠疫耶尔森氏杆菌 |
(20) (4) (4,11) (4) (6,21) (22) |
|
革兰氏阴性杆菌 |
不动杆菌 (也叫鲍曼不动杆菌 军团杆菌 绿脓杆菌 |
(11,15,20) (4) (6,7) |
|
典型细菌 |
拉氏无胆甾原体(支原体) | (23) |
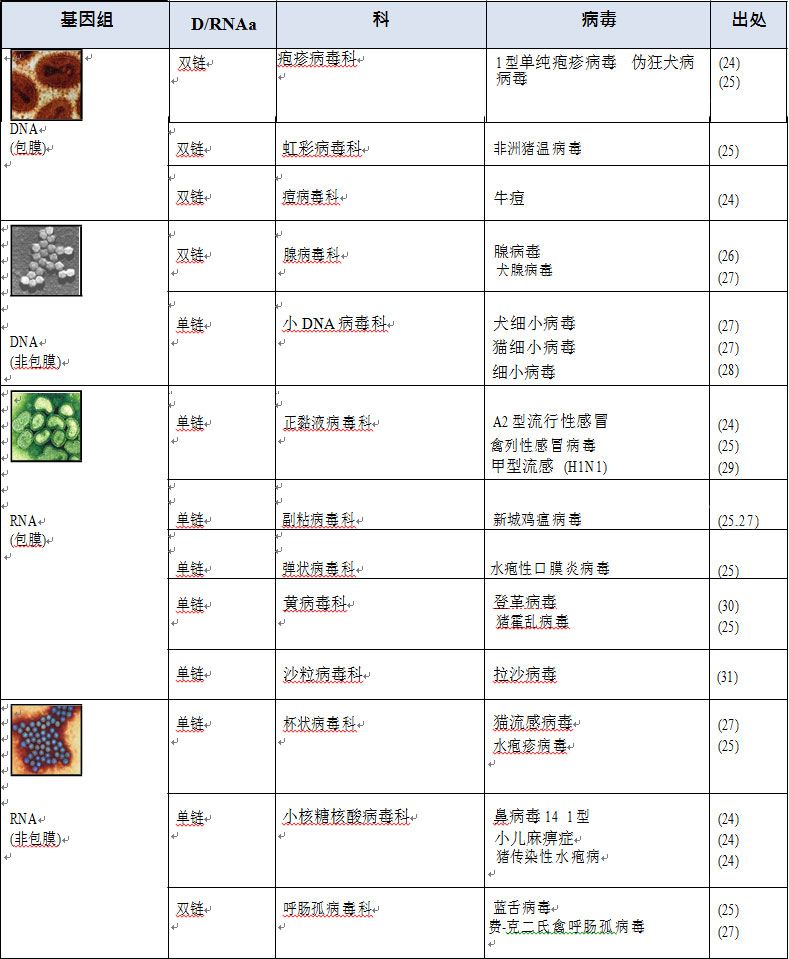
1.2 病毒
1.3 噬菌体

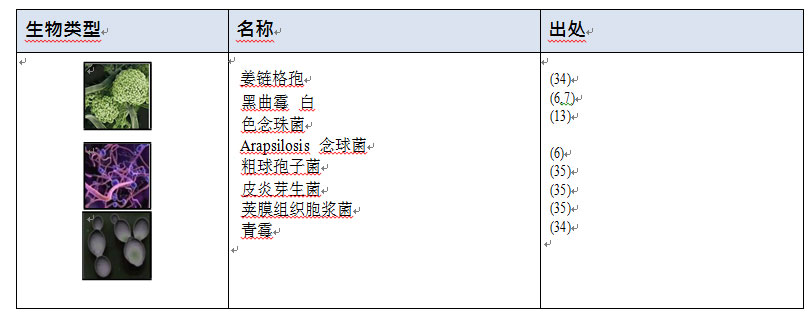
1.4 真菌
1.5 线虫类和原生动物

1.6 其他
证据显示HPV可以灭活蛋白质易传染粒子 (38).
重要研究显示HPV 对断裂的DNA在分子生物学应用中起到至关重要的作用(39).
2. 参考文献/出处
2. 参考文献/出处
Page 6 of 8
12.
Case study from a room bio-decontamination at Imperial College School of Medicine.
2003. Contact Bioquell for further details.
13.
Hall, L., J. A. Otter, J. Chewins, and N. L. Wengenack. 2007. Use of hydrogen peroxide vapor for deactivation of
Mycobacterium tuberculosis in a biological safety cabinet and a room. J.Clin.Microbiol. 45:810-815.
技术报告
HPV生物有效性
Page 7 of 8
14.
French, G. L., J. A. Otter, K. P. Shannon, N. M. Adams, D. Watling, and M. J. Parks.
2004. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J.Hosp.Infect. 57:31-37.
15.
Otter, J. A., M. Cummins, F. Ahmad, van Tonder C., and Y. J. Drabu. 2007. Assessing the biological efficacy and
rate of recontamination following hydrogen peroxide vapour decontamination. J.Hosp.Infect. 67:182-188.
16.
Jeanes, A., G. Rao, M. Osman, and P. Merrick. 2005. Eradication of persistent environmental MRSA.
J.Hosp.Infect. 61:85-86.
17.
Dryden, M., R. Parnaby, S. Dailly, T. Lewis, K. Davis-Blues, J. A. Otter, and A. M. Kearns.
2008. Hydrogen peroxide vapour decontamination in the control of a polyclonal meticillin- resistant Staphylococcus aureus outbreak on a surgical ward. J.Hosp.Infect. 68:190-192.
18.
Determination of the effectiveness of VPHP against methicillin-resistant Staphylococcus aureus,
Staphylococcus epidermidis and Bacillus stearothermophilus. Health Protection Agency (previously Centre for Applied Microbiology and Research),
Porton Down UK. 2001.
19.
Assessment of the efficacy of vapour phase hydrogen peroxide as a room disinfectant. Health Protection Agency
(previously Centre for Applied Microbiology and Research), Porton Down UK. 2003.
20.
Otter, J. A., S. Yezli, M. A. Shouten, A. R. van Zanten, G. Houmes-Zielman, and M. Nohlmans-Paulssen.
2010. Hydrogen peroxide vapor (HPV) decontamination of an intensive care unit to remove environmental reservoirs of multidrug-resistant
Gram-negative rods during an outbreak. Am.J.Infect.Control, accepted.
21.
Bates, C. J. and R. Pearse. 2005. Use of hydrogen peroxide vapour for environmental control during a Serratia
outbreak in a neonatal intensive care unit. J.Hosp.Infect. 61:364-366.
22.
Rogers, J. V., W. R. Richter, M. Q. Shaw, and Y. W. Choi. 2008. Vapour-phase hydrogen peroxide inactivates Yersinia pestis dried on polymers, steel, and glass surfaces. Lett.Appl.Microbiol. 47:279-285.
23.
Otter, J. A., J. Chewins, D. Windsor, and H. Windsor. 2008. Microbiological contamination
in cell culture: a potential role for hydrogen peroxide vapour (HPV)? Cell.Biol.Int. 32:326-327.
24.
Rickloff, J. R. 1990. Use of Vapourized Hydrogen Peroxide for the Bio-decontamination of
Enclosed Areas. Interphex USA Conference. New York.
25.
Heckert, R. A., M. Best, L. T. Jordan, G. C. Dulac, D. L. Eddington, and W. G. Sterritt.
1997. Efficacy of vaporized hydrogen peroxide against exotic animal viruses. Appl.Environ.Microbiol. 63:3916-3918.
26.
Adenovirus deactivation trials. Conducted in commercial confidence. 2003. Contact Bioquell for
further details.
27.
Viral deactivation trials. Conducted in commercial confidence. 2002. Contact Bioquell
for further details.
28.
McDonnell, G., Belete, B., Fritz, C., and Hartling, J. 2001. Room decontamination with vapour hydrogen
peroxide VHP for environmental control of parvovirus. American Association for Laboratory Animal Science (AALAS), Annual meeting. Baltimore, MD.
技术报告
HPV生物有效性
Page 8 of 8
29.
Rudnick, S. N., J. J. McDevitt, M. W. First, and J. D. Spengler. 2009. Inactivating
influenza viruses on surfaces using hydrogen peroxide or triethylene glycol at low vapor concentrations. Am.J.Infect.Control. 37:813-819.
30.
Investigation into the efficacy of hydrogen peroxide vapour in the bio-deactivation of Dengue virus. 2003.
Conducted in commercial confidence. Contact Bioquell for further details.
31.
Otter, J. A., M. Barnicoat, J. Down, D. Smyth, S. Yezli, and A. Jeanes. 2010. Hydrogen peroxide vapor (HPV)
decontamination of an intensive care unit room used to treat a patient with Lassa fever. J.Hosp.Infect.in press.
32.
Otter, J. A. and A. Budde-Niekiel. 2009. Hydrogen peroxide vapor: a novel method for the environmental
control of lactococcal bacteriophages. J.Food.Prot. 72:412-414.
33.
Pottage, T., C. Richardson, S. Parks, J. T. Walker, and A. M. Bennett. 2010. Evaluation of hydrogen peroxide
gaseous disinfection systems to decontaminate viruses. J.Hosp.Infect. 74:55-61.
34.
Information supplied with kind permission of Eli Lilly and Company, Indianapolis, Indiana.
1996.
35.
Hall, L., J. A. Otter, J. Chewins, and N. L. Wengenack. 2008. Deactivation of the dimorphic fungi
Histoplasma capsulatum, Blastomyces dermatitidis and Coccidioides immitis using hydrogen peroxide vapor. Med.Mycol. 46:189-191.
36.
Gustin, E. J., McDonnell, G. E., Mullen, G., and Gordon, B. E. 2002. The efficacy of vapour phase hydrogen
peroxide against nematode infestation: the Caenorhabditis elegans model. American Association for Laboratory Animal Science (AALAS), Annual meeting. San Antonio, Texas, USA
37.
Krause, J. and Riedesel, H. 2002. Elimination of pinworm eggs from caging equipment with vapourised
hydrogen peroxide. Report from the Max-Planck-Institute for experimental medicine. Association for Laboratory Animal Science (AALAS) national meeting. San Antonio, Texas, USA
38.
Fichet, G., K. Antloga, E. Comoy, J. P. Deslys, and G. McDonnell. 2007. Prion inactivation using a new gaseous
hydrogen peroxide sterilisation process. J.Hosp.Infect. 67:278-286.
39.
DNA degradation studies. Conducted in commercial confidence. 2008. Contact Bioquell
for further details.